CTC循環腫瘤細胞檢測
循環腫瘤細胞檢測介紹
一、 癌症轉移是癌症致死的重要原因
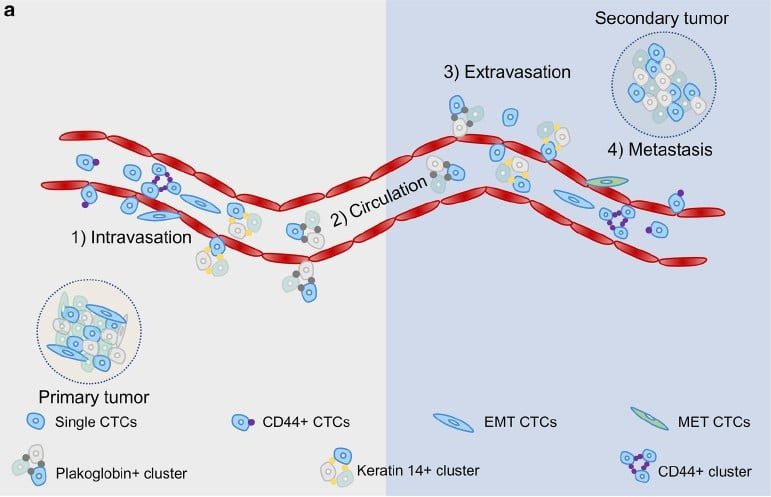
癌症轉移和血液中循環腫瘤細胞 (Circulating Tumor Cell, CTC) 關係密切,轉移的機制主要有以下幾個步驟︓
- 腫瘤細胞會先從原生腫瘤器官組織脫離,穿過微血管到達血管
- 進入血管後的腫瘤細胞,會藉由血液循環運輸到身體各個部位,此時又稱為循環腫瘤細胞
- 當循環腫瘤細胞到達癌症轉移的器官時,會穿透血管管壁,進入該器官組織,形成新腫瘤
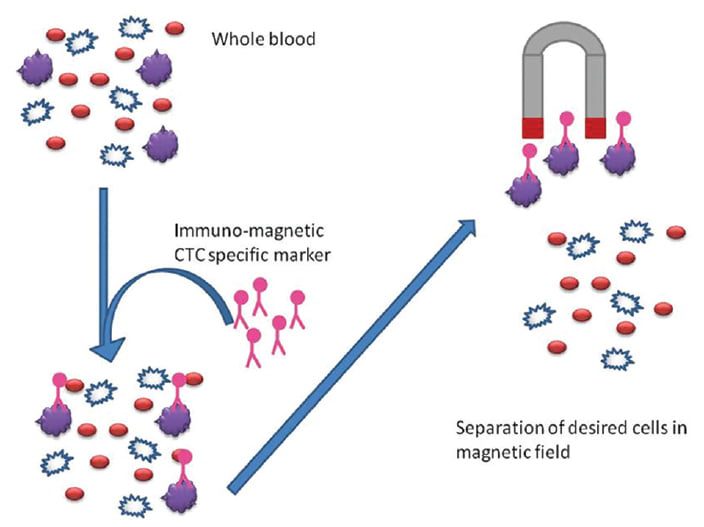
二、 免疫磁性分選是常用循環腫瘤細胞檢測方法
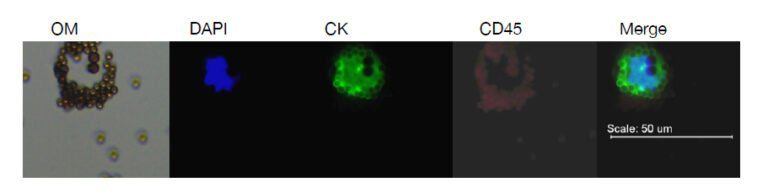
有三個主要步驟分離循環腫瘤細胞,步驟包含採集、富集以及檢測。免疫磁性分選是常用的富集方法,能夠有效從血液中眾多細胞精選出 CTC,常見的標記分子是表皮細胞黏附分子 (簡稱 EpCAM),用來特定標記循環腫瘤細胞,標記有 EpCAM 的微小磁珠會先捕獲帶有 EpCAM 的細胞,利用外加磁力富集這群細胞,再藉由標記其他分子訊號輔助判斷 CTC,像是細胞內角蛋白血球螢光抗體 (CK-FITC) 辨認上皮細胞,白血球細胞螢光抗體 (CD45-Alexa Fluor) 辨識白血球,螢光核染料例如 DAPI 辨別細胞圖像,其中,CTC 被定義為符合腫瘤細胞學形態的特徵並具有 CK-FITC (+), DAPI (+),CD45-Alexa Fluor (-)。
(Potdar PD, Lotey NK. (2015). Role of circulating tumor cells in future diagnosis and therapy of cancer.)
三、 循環腫瘤細胞檢測的新契機與挑戰
CTC 檢測除了作為腫瘤檢測以外,同時也可以作為體內化療藥物藥效的快速評估、病人癒後狀況評估、客製化與精準醫療、健檢等多方應用,然而發展 CTC 時,也面臨了一些挑戰,第一:數量稀少,第二:腫瘤轉移過程會因為上皮間質化(epithelial-mesenchymal transition, 簡稱 EMT) 導致 EpCAM 及 CK 降解,造成 CTC 在捕獲時會有缺失,這也是各個研究團隊正積極解決的問題。
四、總結
儘管 CTC 檢測還有許多問題需要克服,但 CTC 檢測有其優勢,CTC 又稱為液態切片,是一種快速、便利的腫瘤檢測方法,提供患者治療前的輔助診斷,以及治療過程中判斷腫瘤轉移、癒後追蹤、藥效評估等,在臨床被用來作為一種腫瘤檢測的方法。
五、 參考文獻
- Danila, D. C., Pantel, K., Fleisher, M., & Scher, H. I. (2011). Circulating tumors cells as biomarkers: progress toward biomarker qualification. Cancer journal (Sudbury, Mass.), 17(6), 438–450. https://doi.org/10.1097/PPO.0b013e31823e69ac
- Yap, T. A., Lorente, D., Omlin, A., Olmos, D., & de Bono, J. S. (2014). Circulating tumor cells: a multifunctional biomarker. Clinical cancer research : an official journal of the American Association for Cancer Research, 20(10), 2553–2568. https://doi.org/
10.1158/1078-0432.CCR-13-2664 - Alix-Panabières, C., & Pierga, J. Y. (2014). Cellules tumorales circulantes : biopsie liquide du cancer [Circulating tumor cells: liquid biopsy]. Bulletin du cancer, 101(1), 17–23. https:// doi.org/10.1684/bdc.2014.1883
- He, Y., Shi, J., Schmidt, B., Liu, Q., Shi, G., Xu, X., Liu, C., Gao, Z., Guo, T., & Shan, B. (2020). Circulating Tumor Cells as a Biomarker to Assist Molecular Diagnosis for Early Stage Non- Small Cell Lung Cancer. Cancer management and research, 12, 841–854. https://doi.org/ 10.2147/CMAR.S240773
- Lianidou, E. S., & Markou, A. (2011). Circulating tumor cells in breast cancer: detection systems, molecular characterization, and future challenges. Clinical chemistry, 57(9), 1242– 1255. https://doi.org/10.1373/clinchem.2011.165068
- Lee, J. S., Magbanua, M., & Park, J. W. (2016). Circulating tumor cells in breast cancer: applications in personalized medicine. Breast cancer research and treatment, 160(3), 411–424. https://doi.org/10.1007/s10549-016-4014-6
- Tinhofer, I., & Staudte, S. (2018). Circulating tumor cells as biomarkers in head and neck cancer: recent advances and future outlook. Expert review of molecular diagnostics, 18(10),897–906. https://doi.org/10.1080/14737159.2018.1522251
- DiPardo, B. J., Winograd, P., Court, C. M., & Tomlinson, J. S. (2018). Pancreatic cancer
circulating tumor cells: applications for personalized oncology. Expert review of molecular
diagnostics, 18(9), 809–820. https://doi.org/10.1080/14737159.2018.1511429 - Yang, C., Xia, B. R., Jin, W. L., & Lou, G. (2019). Circulating tumor cells in precision oncology: clinical applications in liquid biopsy and 3D organoid model. Cancer cell international, 19, 341. https://doi.org/10.1186/s12935-019-1067-8
- Potdar PD, Lotey NK. (2015). Role of circulating tumor cells in future diagnosis and therapy of cancer. J Cancer Metastasis Treat 2015;1:44-56. http://dx.doi.org/10.4103/2394-4722.158803
【延申閱讀】
- 日本LYMPHOTEC公司影片介紹
- 癌症T細胞免疫療法影片
